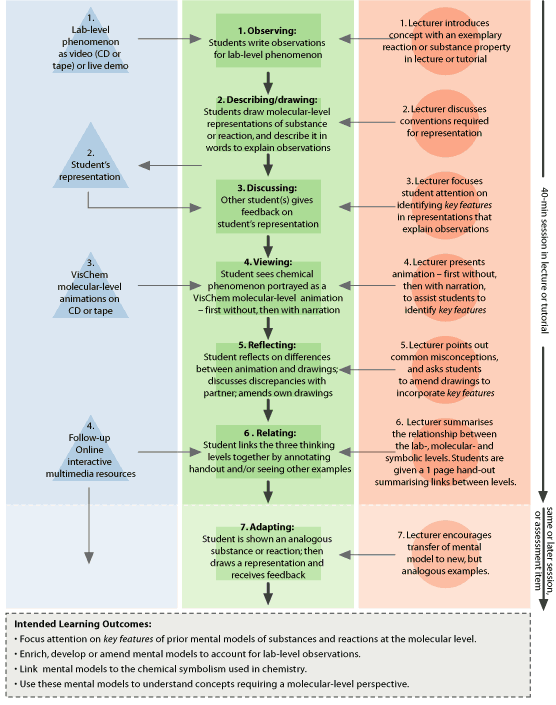
|
ACCESSIBLE RESOURCES
1. Laboratory-Level Video Showing Substance Properties
and Chemical Reactions
Digital video (as QuickTime or AVI movies) showing chemical
reactions is available in supplement packages with most first-year
chemistry textbooks.
These resources are also available in analogue videotape
format. For example, the VisChem “Reactions in Water”
video series:
Tasker R, Bucat R, Sleet R, Chia W, Corrigan D (1996, 1997)
The Molecular World of Reactions in Water, Part 2: Ionic
Equilibrium, Acid/Base and Oxidation/Reduction Chemistry.
The advantage of this video series is that it also includes
most of the VisChem animations.
However, students always prefer live demonstrations, providing
they can see them clearly. This can be done using video-camera
images projected onto large screens in lecture theatres.
2. Students’ Representations
The students’ representations are very valuable learning
resources for other students, and for the lecturer, to identify
alternative conceptions. They can be circulated as frame grabs
from the Molecular-Level Construction Tool (explained below)
or as hand-drawn diagrams.
3. VisChem Molecular-Level Animations
The VisChem animations are available on CD and videotape
formats in the following media:
1. Tasker, R., Bucat, R., Sleet, R., Chia, W., & Corrigan,
D. (1997). VisChem Resources — Learning Chemistry
Through Visualisation of the Molecular Level.
This is a resource CD for chemical educators containing
82 animations in cross-platform QuickTime format (288Mb).
2. Tasker, R., Bucat, R., Sleet, R., Chia, W., Corrigan,
D. (1996, 1997). Molecular World of Water Video (13 min).
The Molecular World of Reactions in Water Part 1: Dissolving,
Precipitation and Complexation Video (25 min). The Molecular
World of Reactions in Water Part 2: Ionic Equilibrium, Acid/Base
and Oxidation/Reduction Chemistry (33 min).
This is a series of three videos, designed for chemical
educators, depicting chemical substances and reactions typically
covered in an introductory chemistry course.
These media are available in Australia and New Zealand from
Video Education Australia, see http://www.vea.com.au,
search for "Reactions in Water", for CD and videos;
and distributed in the US by Films for the Humanities and
Sciences in NTSC video format only, see http://www.films.com,
for item number 7748 with title "The Molecular World
of Reactions upon Water".
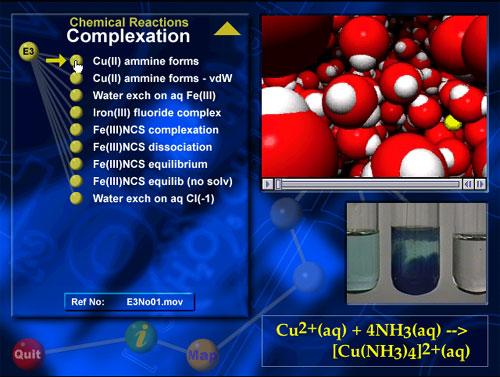
A screen shot illustrating a VisChem animation.
Each VisChem animation on the CD has a reference code so
it can be located and copied into presentation software like
PowerPoint.
The animations are also available in compressed Web-deliverable
versions for use with Acrobat documents on course web sites
(e.g. WebCT). This allows them to be incorporated into online
flexible-learning resources.
4. Interactive Multimedia Support Resources
There are a number of interactive, CD and online support
resources produced with CADRE design and published internationally
to supplement and complement textbooks by WH Freeman &
Co., New York. The WH Freeman web sites are supplements for
their textbooks, but they are not restricted to adopters of
these books. The VisChem animations are presented in the ‘three-thinking-level’
context, often with lab-level video.
Example 1: Tasker, R. (1999). Visualisation
CD for Atkins, P and Jones, L Chemistry: Molecules, Matter
and Change 4th Ed.
This resource comprises an interactive CD supplement with
five major topic areas using VisChem animations, with interactive
questioning, and molecular-level constructions.
Two screen shots are illustrated below.
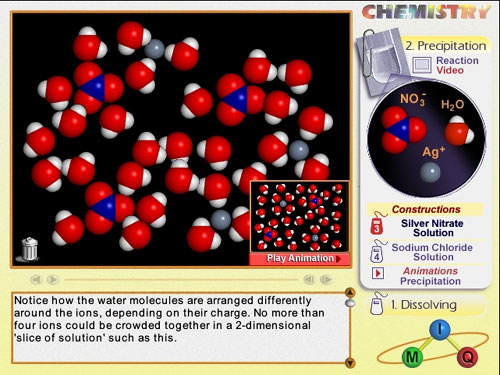
Screen shot of a student's that shows misconceptions
specifically targeted in the animation.
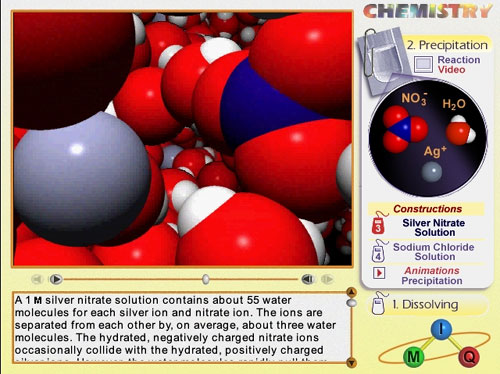
Screen shot showing how an animation is displayed.
Voiceover is also included.
Example 2: Tasker, R. (2002). Web Site.
General Chemistry. An American Chemical Society Project. 1st
Ed.
This resource comprises Web-deliverable modules on selected
topics in each chapter, that use interactive animations and
simulations to develop thinking at the molecular level. The
major emphasis is on molecular-level visualisation.
See
http://www.whfreeman.com/acsgenchemhome/, click on “Tutorials”.
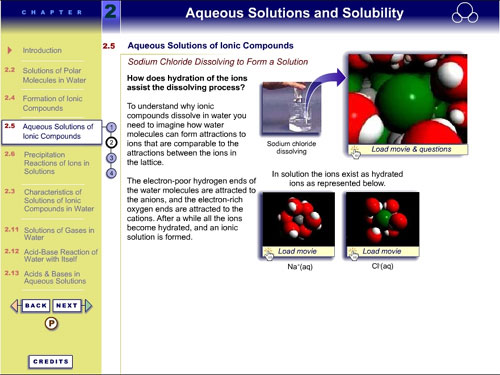
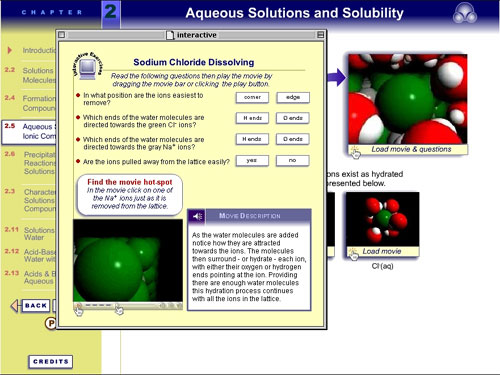
After clicking on the top-right animation frame, the VisChem
animation appears embedded in an interactive interface. Students
are encouraged to engage with the animation by answering questions
and clicking on hotspots:
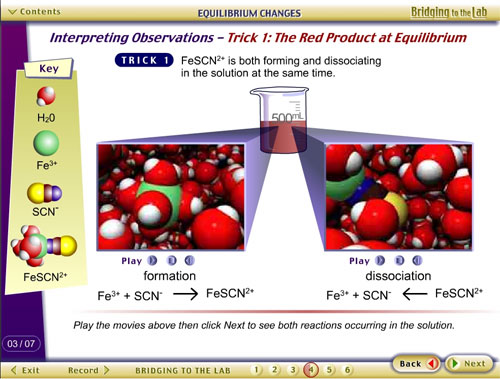
Example 3: Jones, L., & Tasker, R. (2001).
CD and Web Site. Bridging to the Lab: Media Connecting Chemistry
Concepts with Practice.
This resource contains pre- and post-lab modules for selected
laboratory experiments in university-level general chemistry,
containing VisChem animations, with student tracking.
See http://whfreeman.com/bridgingtothelab/.
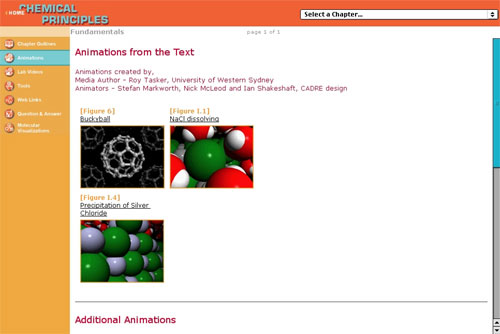
Example 4: Tasker, R. (2001). Web Site for
Atkins P and Jones L Chemical Principles, the Quest for Insight
2nd Ed.
This resource provides web-deliverable versions of VisChem
animations to complement and supplement Figures in the textbook.
These can be downloaded directly from this site.
See http://www.whfreeman.com/chemicalprinciples/
and go to “Animations”.
Example 5: Tasker, R. (2001). CD and Web
Site for ChemCom — Chemistry in the Community. An American
Chemical Society Project. Heikkinen H ed. 4th Ed. Suitable
for upper high-school level.
This resource provides web-deliverable modules using VisChem
animations and interactive graphics to develop thinking at
the molecular level. The major emphasis is on applications
to everyday contexts.
See http://www.whfreeman.com/chemcom
and go to “Interactive ChemCom Media”.
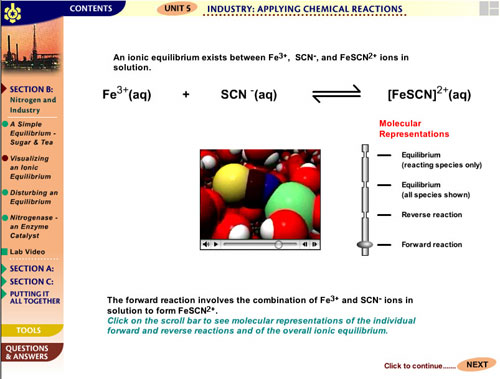
RESOURCES IN CONTEXT
Chemistry involves interpreting visible changes in matter
at the concrete laboratory level (e.g. colour changes, formation
of solids, boiling) in terms of changes in structure and processes
at the invisible molecular level (including atoms and ions).
These changes are then represented at an abstract symbolic
level in two ways: qualitatively, using specialised notation,
terminology, and symbolism; and quantitatively, using mathematics
(equations and graphs).
The need to be able to move between the ‘three thinking
levels’, first described by Johnstone (1991), is a major
problem for students learning chemistry. This learning design
explicitly encourages students to learn new chemistry concepts
by thinking about them at the laboratory, molecular and symbolic
levels.
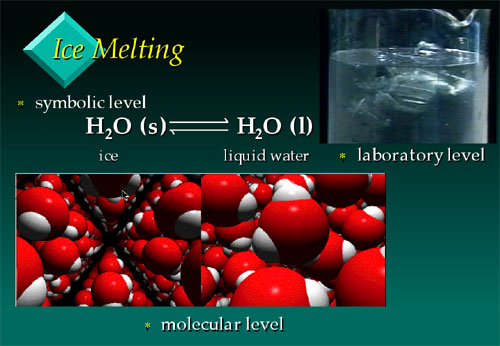
Frame from the VisChem presentation on ice
melting, showing the three ‘thinking’ levels - the
symbolic (chemical equation), laboratory (ice melting in beaker),
and molecular (depicting ice on the left and liquid water
on the right).
Due to a shortage of high quality resources that portray
the molecular level, most chemistry teaching only occurs at
the laboratory and symbolic levels, in the hope that the students’
mental models of the molecular world will ‘develop naturally’.
Students are left to construct these models from the static,
often oversimplified two-dimensional diagrams in textbooks,
or static, often confusing ball-and-stick models, or their
own imagination. However, there is convincing evidence that
most student difficulties and misconceptions in chemistry
stem from inadequate or inaccurate models of the molecular
world.
The aim of the VisChem
project has been to produce multimedia resources (animations,
video, text and sound) to explicitly link the three levels
- the molecular, laboratory, and symbolic - for a variety
of difficult topics in chemistry. The novel resources have
been the Quicktime molecular animations which represent substances
in the solid, liquid, and gaseous states, during phase changes
(e.g. melting), and when they react together. In addition
to a stand-alone format, these animations have also been integrated
into a series of videos that present them in the context of
the three levels.
Reference:
Johnstone, A. H. (1991). Why is Science Difficult to Learn?
Things are Seldom What They Seem. Journal of Computer-Assisted
Learning, 7: 701-70.
VARYING THE RESOURCE SET
The VisChem animations are a core resource because they use
consistent visual conventions over a wide variety of substances
and reactions commonly encountered in first-year chemistry.
However, it is the way these resources are used that is much
more important than the resources themselves.
|